Part 1. Ethical, Legal and Social Implications (ELSI) on the Frontiers of Precision Medicine Research at National Scale: Experiences of the NIH All of Us Research Program
Katherine D. Blizinsky, PhD - National Institutes of Health
ELSIconversations - April 16, 2021
The NIH All of Us Research Program (AoURP) aims to collect genomic, biological, environmental, and lifestyle data longitudinally from one million or more people living in the United States. The AoURP is developing scientific research resources that represent the socioeconomic and demographic diversity of the U.S. population with a focus on increased participation of those historically underrepresented in biomedical research. In this session, panelists will discuss ELSI and policy challenges faced by the AoURP, and detail implementation approaches to address them, drawing upon different stakeholder experiences within the AoURP (policy, program and IRB). The session will highlight how anticipated and unforeseen ELSI challenges for designing a precision medicine research program at national scale are being addressed in a manner that respects the program’s core values while balancing considerations for preventing harm to individuals, groups, and communities. The panelists will first present perspectives on 1) ethical oversight of the research using a single IRB model, 2) challenges with and novel approaches needed for informed consent at scale, 3) balancing ethical tensions in developing and implementing data access and data use policies, and 4) integrating ELSI considerations in policies and governance of biospecimen access and use, respectively. The following moderated open discussion will focus on approaches needed to integrate ELSI research and stakeholder feedback in design and implementation of AoURP and on mechanisms to capture and meet stakeholder expectations going forward, to enable both anticipatory and translational ethics.
Tags
Videos in Series
-
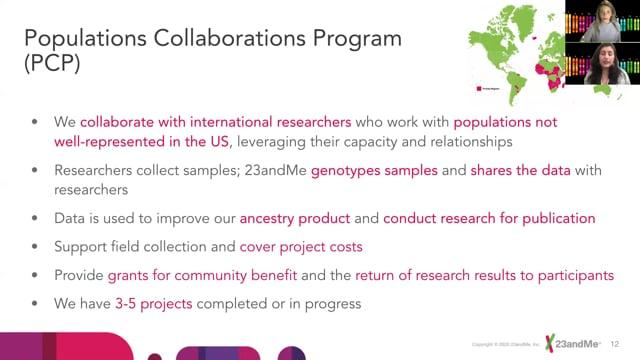
ELSIconversations 1: ELSIcon2020 - A Prospectus on Ethical Issues in the Context of Collaborations Between Academic and Non-academic Institutions on Genetics Research
-

ELSIconversations 1: ELSIcon2020 - Sexual and Gender Minorities’ Perspectives on Genetic Privacy and Identity in Research
-

ELSIconversations 1: ELSIcon2020 - Platform Heals? Ethical Issues in Direct-to-consumer Telepharmacies
-

ELSIconversations 1: ELSIcon2020 - Developing Pathways for Community-led Research with Big Data: A Content Analysis of Stakeholder Interviews
-

ELSIconversations 1: ELSIcon2020 - What does ‘respect for persons’ really mean? Practical considerations for demonstrating respect in genomics research
-

ELSIconversations 1: ELSIcon2020 - A Qualitative Study to Develop a Privacy and Nondiscrimination Best Practice Framework for Personalized Wellness Programs
-

ELSIconversations 1: ELSIcon2020 - Defining the Critical Components of Informed Consent for Genetic Testing
-

ELSIconversations 1: ELSIcon2020 - Part 2. Assessing Access to Care in the Clinical Sequencing Evidence-Generating Research Consortium: Contexts and Challenges
-

ELSIconversations 1: ELSIcon2020 - Patient and family preferences on direct contact by a health system to invite cascade screening
-

ELSIconversations 1: ELSIcon2020 - Part 1. Assessing Access to Care in the Clinical Sequencing Evidence-Generating Research Consortium: Contexts and Challenges
-

ELSIconversations 1: ELSIcon2020 - Which Public, What Comments? An Analysis of Public Comments on Human-Animal Chimera Research Submitted to the National Institutes of Health
-

ELSIconversations 1: ELSIcon2020 - Using an implementation research tool to guide the implementation of non-invasive prenatal screening
-

ELSIconversations 1: ELSIcon2020 - Direct-to-Consumer Genetic Testing: Public Perspectives and Considerations Regarding Ancestry and Kinship
-

ELSIconversations 1: ELSIcon2020 - Human Germline Genome Editing and the Identity Politics of Genetic Disability
-

ELSIconversations 1: ELSIcon2020 - Part 4. Assessing Access to Care in the Clinical Sequencing Evidence-Generating Research Consortium: Contexts and Challenges
-

ELSIconversations 1: ELSIcon2020 - Racial and Ethnic Classification in the Clinic: Is it Just?
-

ELSIconversations 1: ELSIcon2020 - Part 3. Assessing Access to Care in the Clinical Sequencing Evidence-Generating Research Consortium: Contexts and Challenges



