Using an implementation research tool to guide the implementation of non-invasive prenatal screening
Tierry M. Laforce, MA, PhD Candidate - Université de Montréal
ELSIconversations - April 16, 2021
cffDNA screening has changed the landscape of prenatal screening. In Canada, 2 provinces and 1 territory are covering its cost through medicare for women with a high chance of having a fetus with trisomy 21. For the rest of the country, the situation is similar to the one in the United States: some insurers cover it while many women are paying out of pocket. Yet, various stakeholders are calling for universal coverage, not only for trisomy 21, but rather an expanded list of genetic conditions. This presentation will focus on the normative evaluation of the implementation of cffDNA screening. Using research translation as a theoretical framework, we argue that it is currently being implemented as a novel intervention. It needs to be evaluated accordingly before it moves forward. To do so, we are applying the Consolidated Framework for implementation Research (CFIR) to the implementation of cffDNA screening in the Canadian context as a case study. The CFIR is a validated tool used to evaluate contextual factors for a successful implementation of an intervention. It has never been applied to cffDNA screening. The CFIR has been used mostly to analyze the impact of interventions post-implementation. We argue that its strength resides in its ability to identify barriers and facilitators to implementation preemptively. By using the CFIR to identify factors that will influence the implementation of cffDNA screening in practice, this work will help decision-makers select the implementation strategy most likely to be successful with the data emerging from stakeholders.
Tags
Videos in Series
-
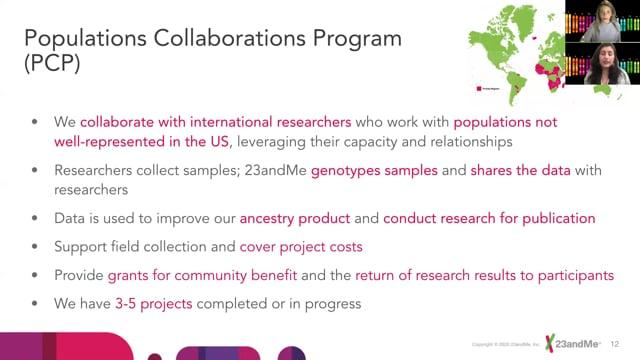
ELSIconversations 1: ELSIcon2020 - A Prospectus on Ethical Issues in the Context of Collaborations Between Academic and Non-academic Institutions on Genetics Research
-

ELSIconversations 1: ELSIcon2020 - Sexual and Gender Minorities’ Perspectives on Genetic Privacy and Identity in Research
-

ELSIconversations 1: ELSIcon2020 - Platform Heals? Ethical Issues in Direct-to-consumer Telepharmacies
-

ELSIconversations 1: ELSIcon2020 - A Qualitative Study to Develop a Privacy and Nondiscrimination Best Practice Framework for Personalized Wellness Programs
-

ELSIconversations 1: ELSIcon2020 - Developing Pathways for Community-led Research with Big Data: A Content Analysis of Stakeholder Interviews
-

ELSIconversations 1: ELSIcon2020 - What does ‘respect for persons’ really mean? Practical considerations for demonstrating respect in genomics research
-

ELSIconversations 1: ELSIcon2020 - Part 4. Assessing Access to Care in the Clinical Sequencing Evidence-Generating Research Consortium: Contexts and Challenges
-

ELSIconversations 1: ELSIcon2020 - Defining the Critical Components of Informed Consent for Genetic Testing
-

ELSIconversations 1: ELSIcon2020 - Part 3. Assessing Access to Care in the Clinical Sequencing Evidence-Generating Research Consortium: Contexts and Challenges
-

ELSIconversations 1: ELSIcon2020 - Patient and family preferences on direct contact by a health system to invite cascade screening
-

ELSIconversations 1: ELSIcon2020 - Part 2. Assessing Access to Care in the Clinical Sequencing Evidence-Generating Research Consortium: Contexts and Challenges
-

ELSIconversations 1: ELSIcon2020 - Which Public, What Comments? An Analysis of Public Comments on Human-Animal Chimera Research Submitted to the National Institutes of Health
-

ELSIconversations 1: ELSIcon2020 - Part 1. Assessing Access to Care in the Clinical Sequencing Evidence-Generating Research Consortium: Contexts and Challenges
-

ELSIconversations 1: ELSIcon2020 - Direct-to-Consumer Genetic Testing: Public Perspectives and Considerations Regarding Ancestry and Kinship
-

ELSIconversations 1: ELSIcon2020 - Human Germline Genome Editing and the Identity Politics of Genetic Disability
-

ELSIconversations 1: ELSIcon2020 - Racial and Ethnic Classification in the Clinic: Is it Just?